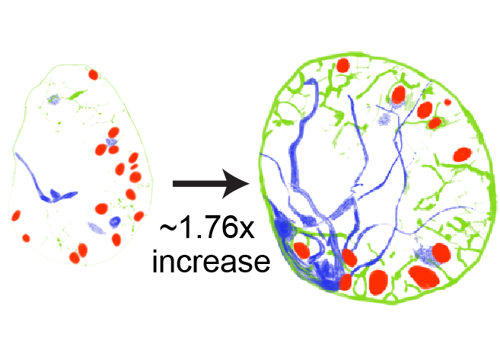
Dietary nutrients provide macromolecules necessary for organism growth and development. In response to animal feeding, evolutionarily conserved growth signaling pathways are activated, leading to increased rates of cell proliferation and tissue growth. It remains unclear how different cell types within developing tissues coordinate growth in response to dietary nutrients and whether coordinated growth of different cell types is necessary for proper tissue function. Here, we report that Drosophila neural stem cells, known as neuroblasts, reactivate from developmental quiescence in a dietary-nutrient-dependent manner. Neuroblast reactivation in the brain requires nonautonomous activation of phosphoinositide 3-kinase (PI3-kinase) signaling from cortex glia and tracheal processes, both of which are closely associated with neuroblasts. Furthermore, PI3-kinase activation in neuroblasts is required nonautonomously for glial membrane expansion and robust neuroblast-glial contact. Finally, PI3-kinase is required cell autonomously for nutrient-dependent growth of neuroblasts, glia, and trachea. Of the 7 Drosophila insulin-like peptides (Dilps), we find that Dilp-2 is required for PI3-kinase activation and growth coordination between neuroblasts and glia in the brain. Dilp-2 induces brain cortex glia to initiate membrane growth and make first contact with quiescent neuroblasts. After contact, neuroblasts increase in size and reenter S-phase. Once reactivated from quiescence, neuroblasts promote growth of cortex glia, which, in turn, form a selective membrane barrier around neuroblasts and their newborn progeny. Our results highlight the importance of bidirectional growth signaling between neural stem cells and surrounding cell types in the brain in response to nutrition and demonstrate how coordinated growth among different cell types drives tissue morphogenesis and function.