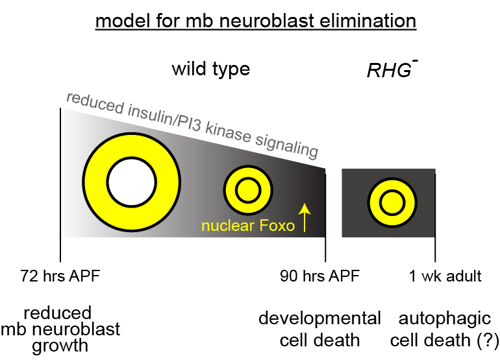
Adult neurogenesis occurs in specific locations in the brains of many animals, including some insects, and relies on mitotic neural stem cells. In mammals, the regenerative capacity of most of the adult nervous system is extremely limited, possibly because of the absence of neural stem cells. Here we show that the absence of adult neurogenesis in Drosophila results from the elimination of neural stem cells (neuroblasts) during development. Prior to their elimination, their growth and proliferation slows because of decreased insulin/PI3 kinase signaling, resulting in nuclear localization of Foxo. These small neuroblasts are typically eliminated by caspase-dependent cell death, and not exclusively by terminal differentiation as has been proposed. Eliminating Foxo, together with inhibition of reaper family proapoptotic genes, promotes long-term survival of neuroblasts and sustains neurogenesis in the adult mushroom body (mb), the center for learning and memory in Drosophila. Foxo likely activates autophagic cell death, because simultaneous inhibition of ATG1 (autophagy-specific gene 1) and apoptosis also promotes long-term mb neuroblast survival. mb neurons generated in adults incorporate into the existing mb neuropil, suggesting that their identity and neuronal pathfinding cues are both intact. Thus, inhibition of the pathways that normally function to eliminate neural stem cells during development enables adult neurogenesis.