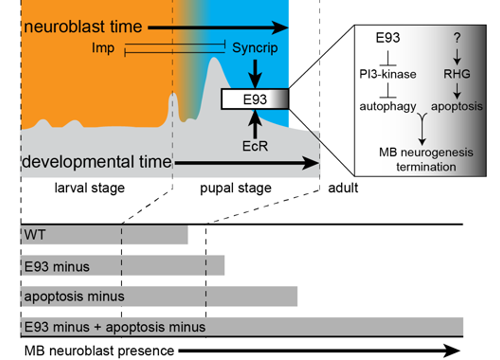
Most neurogenesis occurs during development, driven by the cell divisions of neural stem cells (NSCs). We use Drosophila to understand how neurogenesis terminates once development is complete, a process critical for neural circuit formation. We identified E93, a steroid-hormone-induced transcription factor that downregulates phosphatidylinositol 3-kinase (PI3K) levels to activate autophagy for elimination of mushroom body (MB) neuroblasts. MB neuroblasts are a subset of Drosophila NSCs that generate neurons important for memory and learning. MB neurogenesis extends into adulthood when E93 is reduced and terminates prematurely when E93 is overexpressed. E93 is expressed in MB neuroblasts during later stages of pupal development only, which includes the time when MB neuroblasts normally terminate their divisions. Cell intrinsic Imp and Syp temporal factors regulate timing of E93 expression in MB neuroblasts, and extrinsic steroid hormone receptor (EcR) activation boosts E93 levels high for termination. Imp inhibits premature expression of E93 in a Syp-dependent manner, and Syp positively regulates E93 to promote neurogenesis termination. Imp and Syp together with E93 form a temporal cassette, which consequently links early developmental neurogenesis with termination. Altogether, E93 functions as a late-acting temporal factor integrating extrinsic hormonal cues linked to developmental timing with neuroblast intrinsic temporal cues to precisely time neurogenesis ending during development.