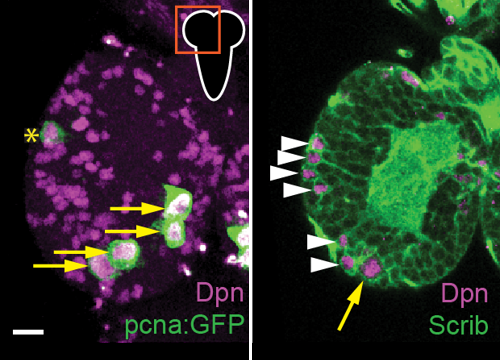
Stem cells enter and exit quiescence as part of normal developmental programs and to maintain tissue homeostasis during adulthood. We report that neural stem cells in Drosophila (neuroblasts) require the evolutionarily conserved Notch cell-cell signaling pathway to enter quiescence during development. When Notch activity is reduced, quiescence is delayed or altogether bypassed, with some neuroblasts dividing continuously during the embryonic to larval transition. Quiescence during this transition allows newborn larvae time to acquire dietary nutrients from their environment in order to restart divisions and continue developmental growth. During embryogenesis before quiescence, neuroblasts express both the Notch receptor and the Notch ligand Delta. During mitosis, Delta is partitioned symmetrically and after division, neuroblast-localized Delta attenuates Notch activity, while GMC daughter-localized Delta activates Notch in neuroblasts. Over time, due to continued GMC production and decreasing Delta levels via Notch-dependent downregulation, the balance of cis-inhibition versus trans-activation shifts leading to increased Notch activity. High Notch inhibits neuroblast cell cycle progression, consequently promoting quiescence while inactivating Notch signaling. Thus, neuroblast quiescence is self-regulated and self-induced, mediated by increased Notch activity and neuroblast divisions.